|
|
|
|
electron; |
neutron; |
proton; |
|
|
|
|
|
 DEE _Radio;
DEE _Radio; |
the highest knowledge level of
modulation (radio) is DEE radio modulation, i.e. beyond XM, FM, AM,
... ; because of
 DEE (Dark Energy Engineering), Gravity as Gun,
Manmade Global Weather, Space crafts, ... ;
because of DEE, Civilization Type can be
changed also;
DEE (Dark Energy Engineering), Gravity as Gun,
Manmade Global Weather, Space crafts, ... ;
because of DEE, Civilization Type can be
changed also; |
|
this DOMAIN
(Satellite DNS System) has mapped all nuclear related ones in our earth;
all nuclear must be demolished in 21st century, and this is a
grace time period; |
think that outer (electron), bounding with inner
(neutron, proton); and then radio (transmit, receive) ... ; |
 |
 |
group |
( |
of |
the |
periodic |
table |
) |
.gif) |
 |
 |
 |
 |
 |
periodic |
table |
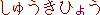 |
; |
|
|
|
| atomic number |
chemical element name |
symbol |
radio active |
isotope |
half life period (t1/2) |
atomic weight |
remark |
remark |
remark |
|
|
like
mutation in bio, positron emission causes (transforming into)
another symbol; m for meta states;
stable with # neutron; |
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| the heaviest
elements (Periodic Table), created by beam of Calcium atoms,
a.k.a. super-heavy elements beyond Element Number 118; Also see:
Calcium;
Chemical _Elements;
Particles; |
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
118
|
O g a n e s s o n |
O g |
|
|
|
294 |
|
|
; |
|
|
294Og; |
|
| 295Og; |
| 296Og; |
| 297Og; |
| 298Og; |
| 300Og; |
| 302Og; |
| |
R n;
O g; U s b; |
|
117
|
T e n n e s s i n e |
Ts |
|
|
|
|
|
|
; |
|
|
293Ts; |
|
| 294Ts; |
| 295Ts; |
| 296Ts; |
| 303Ts; |
| |
At;
Ts; U s u; |
| 116 |
L i v e r m o r i u m |
Lv |
|
|
|
293 |
|
|
; |
| |
286Lv; |
|
| 287Lv; |
| 288Lv; |
| 289Lv; |
| 294Lv; |
| 295Lv; |
| |
Po;
Lv; U s n; |
|
115
|
M o s c o v i u m |
Mc |
|
|
|
289 |
|
|
; |
|
|
290Mc; |
|
| 291Mc; |
| |
Bi;
Mc; U h e; |
| 114 |
F l e r o v i u m |
Fl |
|
|
|
289 |
|
|
; |
| |
291Fl; |
|
| 292Fl; |
| 298Fl; |
| 310Fl; |
| |
P b;
Fl; U h o; |
|
113
|
N i h o n i u m |
N h |
|
|
|
286 |
|
|
; |
|
|
278Nh; |
#
millisecond |
|
|
|
|
| 282Nh; |
#
millisecond |
|
|
|
|
| 283Nh; |
#
second |
|
|
|
|
| 284Nh; |
#
second |
|
|
|
|
| 285Nh; |
#
second |
|
|
|
|
| 286Nh; |
|
| 287Nh; |
#
minute |
|
|
|
|
| |
T l;
N h; U h s; |
| 112 |
C o p e r n i c i u m |
Cn |
|
|
|
285 |
|
|
; |
| |
277Cn; |
|
| 281Cn; |
# millisecond |
|
|
|
; |
| 284Cn; |
# millisecond |
|
|
|
; |
| 285Cn; |
# second |
|
|
|
; |
| 291Cn; |
# year |
|
|
|
; |
| 293Cn; |
# year |
|
|
|
; |
| |
Hg;
Cn; U h h; |
| 111 |
R o e n t g e n i u m |
R g |
|
|
|
282 |
|
|
; |
| |
272Rg; |
|
| 282Rg; |
# minute |
|
|
|
; |
| 283Rg; |
|
| |
Au;
R g; U h p; |
| 110 |
D a r m s t a d t i u m |
Ds |
|
|
|
281 |
|
|
; |
| |
267Ds; |
|
| 270Ds; |
| 271Ds; |
| 280Ds; |
| 294Ds; |
| |
Pt;
Ds; U h q; |
|
109
|
M e i t n e r i u m |
Mt |
|
|
|
268 |
|
|
; |
| |
|
|
108
|
H a s s i u m |
Hs |
|
|
|
265 |
|
|
; |
| |
|
|
107
|
B o h r i u m |
B h |
|
|
|
264 |
|
|
; |
| |
|
|
106
|
S e a b o r g i u m |
Sg |
|
|
|
266 |
|
|
; |
| |
|
|
105
|
D u b n i u m |
Db |
|
|
|
262 |
|
|
; |
| |
|
|
104
|
R u t h e r f o r d i u m |
R f |
|
|
|
261 |
|
|
; |
| |
|
|
103
|
Lawrencium |
L r |
|
|
|
262 |
|
|
; |
| |
|
|
102
|
Nobelium |
No |
|
|
|
259 |
|
|
; |
| |
|
|
101
|
Mendelevium |
M d |
|
|
|
258 |
|
|
; |
| |
|
|
100
|
Fermium |
Fm |
|
|
|
257 |
|
|
; |
| |
|
|
99
|
Einsteinium |
Es |
|
|
|
252 |
|
|
; |
| |
|
|
98
|
Californium |
C f |
|
|
|
251 |
|
|
; |
| |
|
|
97
|
Berkelium |
B k |
|
|
|
247 |
|
|
; |
| |
|
|
96
|
Curium |
Cm |
|
|
|
247 |
|
|
; |
| |
|
|
95
|
Americium |
Am |
|
|
|
243 |
|
|
; |
| |
|
|
94
|
Plutonium |
P u |
|
|
|
244 |
|
|
; |
| |
|
|
93
|
Neptunium |
N p |
|
|
|
237 |
|
|
; |
| |
|
|
92
|
Uranium |
U |
|
|
|
238.0 |
|
|
; |
| |
|
|
91
|
Protactinium |
Pa |
|
|
|
231.0 |
|
|
; |
| |
|
|
90
|
Thorium |
T h |
|
|
|
232.0 |
|
|
; |
| |
|
|
89
|
Actinium |
Ac |
|
|
|
227 |
|
|
; |
| |
|
|
88
|
Radium |
Ra |
|
|
|
226 |
|
|
; |
| |
|
|
87
|
Francium |
Fr |
|
|
|
223 |
|
|
; |
| |
|
|
86
|
Radon |
R n |
|
|
|
222 |
|
|
; |
| |
|
|
85
|
Astatine |
At |
|
|
|
210 |
|
|
; |
| |
|
|
84
|
Polonium |
Po |
|
|
|
210 |
|
|
; |
| |
|
|
83
|
Bismuth |
Bi |
|
|
|
208.9 |
|
|
; |
| |
|
|
82
|
Lead |
P b |
|
|
|
207.2 |
|
|
; |
| |
|
|
81
|
Thallium |
T l |
|
|
|
204.3 |
|
|
; |
| |
|
|
80
|
Mercury |
Hg |
|
|
|
200.5 |
|
|
; |
| |
|
|
79
|
Gold |
Au |
|
|
|
196.9 |
|
|
; |
| |
gold; gold coin (i.e. valuable money), highest
value;
 Radical200;
Radical200;
|
|
gold;
 Radical240;
Radical240;
|
| |
|
78
|
Platinum |
Pt |
|
|
|
195.0 |
|
|
; |
| |
|
|
77
|
Iridium |
I r |
|
|
|
192.2 |
|
|
; |
| |
|
|
76
|
Osmium |
Os |
|
|
|
190.2 |
|
|
; |
| |
|
|
75
|
Rhenium |
Re |
|
|
|
186.2 |
|
|
; |
| |
|
|
74
|
Tungsten |
W |
|
|
|
183.8 |
|
|
; |
| |
|
|
73
|
Tantalum |
Ta |
|
|
|
108.9 |
|
|
; |
| |
|
|
72
|
Hafnium |
H f |
|
|
|
178.4 |
|
|
; |
| |
|
|
71
|
Lutetium |
Lu |
|
|
|
174.9 |
|
|
; |
| |
|
|
70
|
Ytterbium |
Y b |
|
|
|
173.0 |
|
|
; |
| |
|
|
69
|
Thulium |
Tm |
|
|
|
168.9 |
|
|
; |
| |
|
| 68 |
Erbium |
E r |
|
|
|
167.2 |
|
|
; |
| |
|
|
67
|
Holmium |
Ho |
|
|
|
164.9 |
|
|
; |
| |
|
|
66
|
Dysprosium |
D y |
|
|
|
162.5 |
|
|
; |
| |
|
| 65 |
Terbium |
Tb |
|
|
|
158.9 |
|
|
; |
| |
|
|
64
|
Gadolinium |
G d |
|
|
|
157.2 |
|
|
; |
| |
ferromagnetic;
|
|
gadolinium (3) ion; |
|
63
|
Europium |
E u |
|
|
|
151.9 |
|
|
; |
| |
|
|
62
|
Samarium |
S m |
|
|
|
150.3 |
|
|
; |
| |
|
|
61
|
Promethium |
Pm |
|
|
|
145 |
|
|
; |
| |
|
|
60
|
Neodymium |
N d |
|
|
|
144.2 |
|
|
; |
| |
|
|
59
|
Praseodymium |
Pr |
|
|
|
140.9 |
|
|
; |
| |
|
|
58
|
Cerium |
C e |
|
|
|
140.1 |
|
|
; |
| |
|
|
|
|
|
|
|
|
|
|
57
|
Lanthanum |
La |
|
|
|
138.9 |
|
|
; |
| |
|
|
56
|
Barium |
B a |
|
|
|
137.3 |
|
|
; |
| |
|
|
55
|
Cesium |
Cs |
|
|
|
132.9 |
|
|
; |
| |
|
|
54
|
Xenon |
X e |
|
|
|
131.2 |
|
|
; |
| |
124Xe; |
|
| |
|
129Xe; |
| |
130Xe; |
| |
131Xe; |
# second |
|
; |
| |
|
133Xe; |
|
| |
134Xe; |
| |
|
135Xe; |
|
|
thermal
neutrons; |
| |
136Xe; |
|
| |
Kr;
X e; R n; |
|
53
|
Iodine |
I |
|
|
|
126.9 |
|
; |
; |
| |
123I; |
# hour |
|
; |
electron capture; |
gamma radiation; |
| nuclear
medicine; |
| |
|
125I; |
# day |
|
;
radiation therapy; |
treat
brain tumor;
treat prostate cancer;
treat u v e a l melanoma; |
| |
127I; |
|
| 129I; |
# year |
|
; |
| 131I; |
# day |
|
; |
beta decay; |
| |
|
|
|
treat
thyroid cancer; |
| |
Br;
I; At; |
|
52
|
Tellurium |
Te |
|
|
|
127.6 |
|
; |
; |
| |
|
|
|
; |
; |
| |
105Te; |
|
|
nuclear isomer; |
| 106Te; |
|
|
alpha decay; |
| 110Te; |
|
|
| 120Te; |
|
| 122Te; |
| 123Te; |
| 124Te; |
| 125Te; |
| 126Te; |
| |
|
128Te; |
# year |
|
; |
| 130Te; |
# year |
|
; |
| |
142Te; |
|
|
nuclear
isomer; |
| |
Se;
Te; Po; |
|
51
|
Antimony |
S b |
|
|
|
121.7 |
|
; |
; |
| |
120m1Sb; |
# day |
|
; |
| 121Sb; |
|
| 123Sb; |
|
|
beta
decay; |
| 125Sb; |
# year |
|
; |
| |
meta
stable states; |
|
As;
S b; Bi; |
|
50
|
Tin |
Sn |
|
|
|
118.7 |
; |
; |
; |
| |
|
100Sn; |
|
| |
111Sn; |
|
|
meta stable isomer; |
| 112Sn; |
|
|
| 113Sn; |
|
|
| 114Sn; |
|
|
protons; |
| 115Sn; |
|
|
| 116Sn; |
|
|
slow neutron capture; |
| 118Sn; |
|
|
| 120Sn; |
|
|
| 121mSn; |
# year |
|
|
| 122Sn; |
|
|
rapid neutron capture; |
| 124Sn; |
|
|
| |
|
132Sn; |
|
| |
nuclear
physics magic number (50); |
|
G e;
S n; P b; |
|
49
|
Indium |
In |
|
|
|
114.8 |
; |
; |
; |
| |
97In; |
|
| 111In; |
# day |
|
; |
| 113In; |
|
| 114mIn; |
# day |
|
; |
| 115In; |
# year |
|
; |
electron capture;
positron emission; |
| 135In; |
|
| |
meta
states; |
|
G a;
In; T l; |
| 48 |
Cadmium |
Cd |
|
|
|
112.4 |
; |
; |
; |
| |
95Cd; |
|
| 106Cd; |
|
|
double electron capture; |
| 108Cd; |
|
|
| 109Cd; |
# day |
|
; |
| 110Cd; |
|
| 111Cd; |
| 112Cd; |
| |
|
113Cd; |
# year |
|
; |
beta decay; |
| 116Cd; |
# year |
|
; |
2 neutrino; double
beta decay; |
| |
113mCd; |
# year |
|
; |
| 114Cd; |
|
|
double beta decay; |
| 115Cd; |
# hour |
|
; |
| 115mCd; |
# day |
|
; |
| 117mCd; |
# hour |
|
; |
| 132Cd; |
|
| |
Zn;
Cd; Hg; |
|
47
|
Silver |
Ag |
|
|
|
107.8 |
|
|
; |
| |
93Ag; |
|
| 105Ag; |
# day |
|
; |
| 107Ag; |
|
|
electron
capture; |
| |
|
108mAg; |
# year |
|
; |
| |
109Ag; |
|
| 111Ag; |
# day |
|
; |
| 112Ag; |
# hour |
|
; |
| 130Ag; |
|
| |
iron meteorite; |
|
Cu;
Ag; Au; |
|
46
|
Palladium |
Pd |
|
|
|
106.4 |
|
; |
; |
| |
91Pd; |
|
| 100Pd; |
# day |
|
; |
| 101Pd; |
# hour |
|
; |
| 103Pd; |
# day |
|
; |
| 106Pd; |
|
electron
capture; |
| 107Pd; |
# year |
|
; |
| 109Pd; |
# hour |
|
; |
| 112Pd; |
# hour |
|
; |
| 123Pd; |
|
| |
Ni;
Pd; Pt; |
| 45 |
Rhodium |
R h |
|
|
|
102.9 |
|
; |
; |
| |
93Rh; |
|
| 99Rh; |
# day |
|
; |
| 100Rh; |
# hour |
|
; |
| 101Rh; |
# year |
|
; |
| 101mRh; |
# day |
|
; |
# M eV |
; |
| 102Rh; |
# day |
|
; |
| 102mRh; |
# year |
|
; |
# M eV |
; |
| 102mRh; |
# year |
|
; |
| 103Rh; |
|
| 117Rh; |
| |
Co;
R h; I r; |
| 44 |
Ruthenium |
Ru |
|
|
|
101.0 |
|
; |
; |
| |
90Ru; |
|
| 95Ru; |
# hour |
|
; |
| 101Ru; |
|
stable with # neutrons; |
| 102Ru; |
electron capture; |
| 104Ru; |
stable with #
neutrons; |
| 105Ru; |
# hour |
|
; |
| 115Ru; |
|
| |
Fe;
Ru; Os; |
| 43 |
Technetium |
T c |
|
|
|
98 |
|
|
; |
| |
neutron capture;
uranium ore; |
| |
99mTc; |
|
gamma ray nuclear
isomer; nuclear medicine; |
| |
M n;
T c; Re; |
| 42 |
Molybdenum |
Mo |
|
|
|
95.9 |
|
; |
; |
| |
Cr;
Mo; W; |
|
41
|
Niobium |
N b |
|
|
|
92.9 |
|
; |
; |
| |
C b |
|
|
|
|
; |
formerly |
; |
| |
V;
N b; Ta; |
| superconducting; |
| 40 |
Zirconium |
Z r |
|
|
|
91.2 |
|
; |
; |
| |
83Zr;
85Zr; |
|
positron emission; |
83mZr;
85mZr;
90m1Zr;
90m2Zr;
91mZr; |
# millisecond |
; |
meta stable isomer; |
| 88Zr; |
|
electron capture; |
| 89mZr; |
# minute |
; |
meta stable isomer; |
90Zr;
91Zr;
92Zr;
94Zr; |
|
| 93Zr; |
|
|
electron
emission; |
| |
|
96Zr; |
|
| |
110Zr; |
# millisecond |
; |
| |
Ti;
Z r; H f; |
|
39
|
Yttrium |
Y |
|
|
|
88.9 |
|
; |
; |
| |
89Y; |
|
| |
Sc;
Y; Lu; |
|
38
|
Strontium |
S r |
|
|
|
87.6 |
; |
; |
; |
| |
90Sr; |
|
| |
Ca;
S r; B a; |
|
37
|
Rubidium |
R b |
|
|
|
85.4 |
|
; |
; |
| |
K;
R b; Cs; |
|
36
|
Krypton |
Kr |
|
|
|
83.7 |
|
|
; |
| |
A r;
Kr; X e; |
| Krypton ion (gas
laser); 605 nm wavelength; |
|
35
|
Bromine |
Br |
|
|
|
79.9 |
|
; |
; |
| |
Cl;
Br; I; |
| Bromine
ion (Br-, Br minus); |
|
34
|
Selenium |
Se |
|
|
|
78.9 |
; |
; |
; |
| |
S;
Se; Te; |
|
33
|
Arsenic |
As |
|
|
|
74.9 |
|
|
; |
| |
P;
As; S b; |
|
32
|
Germanium |
G e |
|
|
|
72.6 |
; |
|
; |
| |
Si;
G e; S n; |
|
31
|
Gallium |
G a |
|
|
|
69.7 |
|
; |
; |
| |
Al;
G a; In; |
| Gallium
(3); |
|
30
|
Zinc |
Zn |
|
|
|
65.3 |
|
; |
; |
| |
minus;
Zn;
Cd; |
|
copper (Cu) bronze metal;
 Radical188;
Radical188; |
|
29
|
Copper |
Cu |
|
|
|
63.5 |
; |
; |
; |
| |
minus;
Cu;
Ag; |
| % of copper per body
weight;
bone; liver; muscle; |
|
28
|
Nickel |
Ni |
|
|
|
58.6 |
|
; |
|
| |
58Ni;
64Ni; |
|
| |
minus;
Ni;
Pd; |
|
ferromagnetic; |
|
Ni 58 isotope and Ni 64 isotope are having the same chemical
property and electrical property, but 6 neutrons different make them weight
diff; Also see: oscillations diff in parallel plates; |
| 27 |
Cobalt |
Co |
|
|
|
58.9 |
; |
; |
; |
| |
minus;
Co;
R h; |
|
ferromagnetic; |
 |
iron |
content |
|
|
|
|
|
|
|
| 26 |
Iron |
Fe |
|
|
|
55.8 |
|
|
; |
| |
minus;
Fe; Ru; |
|
Distribution (Iron) via blood proteins, bone marrow,
enzymes, f e r r i t i n, hemoglobin, muscle, plasma transportation,
tissue, ... ; |
|
F e r r;
Ferro; ferromagnetic; |
|
Lo Shu Magic Square topology, Sudoku, C
Sequence Number (directional) Gene Therapy System, also see:
Network Topology;
|
| 25 |
Manganese |
M n |
|
|
|
54.9 |
|
|
; |
| |
minus;
M n; T c; |
| manganese (2) ion;
enzymes; |
| 24 |
Chromium |
Cr |
|
|
|
51.9 |
; |
|
; |
| |
minus;
Cr; Mo; |
| chromium
ion (Cr (3)); |
| chromium ion (Cr (5))
toxic; |
| 23 |
Vanadium |
V |
|
|
|
50.9 |
; |
; |
; |
| |
minus;
V; N b; |
| 22 |
Titanium |
Ti |
|
|
|
47.8 |
|
|
; |
| |
46Ti;
47Ti;
48Ti;
49Ti;
50Ti; |
|
| |
minus;
Ti; Z r; |
|
lightweight alloy; |
| 21 |
Scandium |
Sc |
|
|
|
44.9 |
|
; |
; |
| |
minus;
Sc; Y; |
| 20 |
Calcium |
Ca |
|
|
|
40.0 |
; |
; |
|
| |
Mg;
Ca; S r; |
| calcium
ion; |
| |
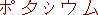 |
|
|
| 19 |
Potassium |
K |
|
|
|
39.0 |
; |
; |
|
| |
Na;
K; R b; |
|
potassium ion; potassium ion (ECG) abnormal; |
| 18 |
Argon |
A r |
|
|
|
39.9 |
|
|
; |
| |
Ne;
A r; Kr; |
| 17 |
Chlorine |
Cl |
|
|
|
35.4 |
|
; |
; |
| |
F;
Cl; Br; |
| 16 |
Sulfur |
S |
|
|
|
32.0 |
|
; |
; |
| |
O;
S; Se; |
| 15 |
Phosphorus |
P |
|
|
|
30.9 |
; |
; |
; |
| |
N;
P; As; |
| 14 |
Silicon |
Si |
|
|
|
28.08 |
; |
; |
; |
| |
C;
Si; G e; |
| silicon
dioxide (silica); |
| sand's
sizes may vary, depending upon planet's size, ... ; |
| 13 |
Aluminum |
Al |
|
|
|
26.9 |
|
; |
; |
| |
B;
Al; G a; |
| 12 |
Magnesium |
Mg |
|
|
|
24.3 |
|
; |
|
| |
Be;
Mg; Ca; |
|
magnesium ion (ATP, DNA, RNA); 300+ enzymes; |
| 11 |
Sodium |
Na |
|
|
|
22.9 |
|
; |
|
| |
Li;
Na; K; |
| sodium
ion e.g. Extra Cellular Fluid (E C F); |
| 10 |
Neon |
Ne |
|
|
|
20.1 |
|
|
; |
| |
He;
Ne; A r; |
| 9 |
Fluorine |
F |
|
|
|
18.9 |
|
|
; |
| |
minus;
F; Cl; |
|
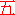 5;
5;
WHY five is here to define sir ? well trained
kids ! beyond 1, 2, 3 (123, a b c in
numerological), 4 is like
Numbers in Dhamma (4 Novel Truths),
and our
Solar System can be defined ((13 = 8 + 5) a.k.a.
secant & segment of our universes) and 4th factor is like heat
sensing (also see:
Schematic Symbol), And Then, beyond
Oxygen, e.g. Origin of Sound,
e.g. very very very far away in distance, beyond 1234, And Then,
it's necessary to refreshed and linked 5 ... ; because this
DOMAIN 's contents are intended to be using in
ACT2 and
ACT3
imaginary hyper space
... ; |
| |
 |
|
|
|
|
|
|
|
|
| 8 |
Oxygen |
O |
|
|
|
15.9 |
|
|
; |
 |
oxygen |
NMR |
; |
|
|
Also see:
o G T S U; |
| |
minus;
O; S; |
|
O2- Negative Ion (s),
plasma cluster
Ion; Airborne water vapor; OH radicals; IFF PMD patterns, also see:
P vector; O3, ozone; |
 Don't you
ever think that the one and only the
sun is something
related to O, Don't you
ever think that the one and only the
sun is something
related to O,  WHERE 1 is sun, 2 is known as anomaly
an angular degree to outer planet, [3 to
1 dimension has been suggested], 3 is known as a p sides line, 4 is
4 planet prediction,
5 is this DOMAIN 's you and me tactic to keep 5
s i l a, 6 is a little crack to be
a n a t a again because WHERE 1 is sun, 2 is known as anomaly
an angular degree to outer planet, [3 to
1 dimension has been suggested], 3 is known as a p sides line, 4 is
4 planet prediction,
5 is this DOMAIN 's you and me tactic to keep 5
s i l a, 6 is a little crack to be
a n a t a again because
 WHEN the time in
documents such as 10 dynasties in China, Chinese people would think that never
ending dynasty and technology, 7 is known as perihelion, and 8 is known as
aphelion. Also see: Yin & Yang with Water; Important
element
fire; WHEN the time in
documents such as 10 dynasties in China, Chinese people would think that never
ending dynasty and technology, 7 is known as perihelion, and 8 is known as
aphelion. Also see: Yin & Yang with Water; Important
element
fire; |
| |
 |
|
|
|
|
|
|
|
|
| 7 |
Nitrogen |
N |
|
|
|
14.0 |
|
|
; |
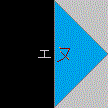 |
nitrogen |
NMR |
; |
|
|
Also see:
n G T S U; |
| |
minus;
N; P; |
| Liquid nitrogen, for cooling, i.e. -80 °C inside
C C D s in Sloan Survey, for
online digital astronomical images, in
ACT 1 . self wonder stage; |
|
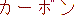 carbon;
carbon; |
|
our earth's soil (organic carbon content), measured by (at 1
meter depth, 1000 kilometer scale) distance based, average is
between (10 ~ 25) kg m-2,
and (25 ~ 50) kg m-2;
|
| 6 |
Carbon |
C |
|
|
|
12.0 |
|
|
; |
| |
12C;
13C;
14C; |
|
May 31, 2024; a
new Graphene material (potentials in diff color codes (iroLED);
Usage (graphene), derived from "graphite,"
doko
 WHERE single atom layer of (6, Carbon,
C)
atoms; Also see: Materials;
WHERE single atom layer of (6, Carbon,
C)
atoms; Also see: Materials; |
| |
minus;
C; Si; |
| 5 |
Boron |
B |
|
|
|
10.8 |
|
|
; |
| |
minus;
B; Al; |
| 4 |
Beryllium |
Be |
|
|
|
9.0 |
|
|
; |
| |
minus;
Be; Mg; |
| 3 |
Lithium |
Li |
|
|
|
6.9 |
|
|
; |
| |
H;
Li; Na; |
| 2 |
Helium |
He |
|
|
|
4.0 |
|
|
; |
| |
minus;
He; Ne; |
 |
hydrogen |
|
|
|
|
|
|
|
|
 |
hydrogen |
|
|
|
|
|
|
|
|
 |
hydrogen |
|
|
|
|
|
|
|
|
 |
hydrogen |
|
|
|
|
|
|
|
|
| |
 |
|
|
|
|
|
|
|
|
| 1 |
Hydrogen |
H |
|
|
|
1.007 94 u |
|
|
; |
 |
hydrogen |
NMR |
; |
|
|
Also see:
h G T S U; |
| |
minus;
H; Li; |
H+ Positive
 Ion (s),
plasma cluster
Ion; Airborne water vapor; OH radicals; IFF PMD patterns, also see:
P vector; Ion (s),
plasma cluster
Ion; Airborne water vapor; OH radicals; IFF PMD patterns, also see:
P vector; |
| IFF gas-hydrogen emits spectral lines; Also see: visible gas/cloud lines in the
sky
"in the air,
airborne, either can be visible at low altitude above sea level AND/OR can be
visible at high altitude below earth's atmosphere", since 1990s,
still one of the military top secrets, formula not available to public;
p-b r a n e s proliferation in M Theory;
2 H+, 2 hydrogen ions;
|
|
|
![]() ;
;
![]() ;
;
![]() ;
;
;
![]() ; Updated in
; Updated in
2024 :
12 :
16 :
![]() Periodic Table ;
Periodic Table ;
![]() energy i.e. from Methane Hydrate (cold & frozen) to Natural Gas;
energy i.e. from Methane Hydrate (cold & frozen) to Natural Gas; ![]() HOW
formulation procedures of
symbols
define Civilization Type (e.g.
ACT1,
ACT2,
ACT3),
and the highest Civilization Type (e.g. ACT3) means knowing all
symbols
HOW
formulation procedures of
symbols
define Civilization Type (e.g.
ACT1,
ACT2,
ACT3),
and the highest Civilization Type (e.g. ACT3) means knowing all
symbols
![]() HOW to create;
therefore, some symbols are restricted info, some symbols are unknown (not
documented), some symbols are ... ;
HOW to create;
therefore, some symbols are restricted info, some symbols are unknown (not
documented), some symbols are ... ;
![]() (
(![]() bio;
bio;
![]() density;
density;
![]() gas;
gas;
electricity;
form (e.g.
beats, bubbles, crystal, jelly, liquid, powder, solid, ... );
![]() heat;
heat;
light;
![]() magnet;
magnet;
![]() pressure;
pressure;
temperature;
![]() type
(e.g. appearance, category, color, electron environment, phase, ... ));
type
(e.g. appearance, category, color, electron environment, phase, ... ));
Air, our earth) : (Argon (
![]() A r), Carbon dioxide (
A r), Carbon dioxide (![]() CO2), Carbon monoxide (
CO2), Carbon monoxide (![]() CO), Helium (
CO), Helium (![]() He), Hydrogen (
He), Hydrogen (![]() H2), Krypton (
H2), Krypton (![]() Kr), Methane (
Kr), Methane (![]() CH4), Neon (
CH4), Neon (![]() Ne), Nitrous oxide (
Ne), Nitrous oxide (![]() N2O), Oxygen (
N2O), Oxygen (![]() O2), Ozone (
O2), Ozone (![]() O3), Nitrogen (
O3), Nitrogen (![]() N2), Water vapor (
N2), Water vapor (![]() H2O)) our earth ... ;
H2O)) our earth ... ;